Results 11 to 17 of 17
Thread: Glycerin on hones
-
01-27-2017, 05:30 AM #11Senior Member

- Join Date
- Sep 2015
- Posts
- 695
Thanked: 77
I've never tried glycerin but now you all have me curious. The biggest problem I'm have in currently with oil is finding a very thin oil for something such as my Lynn idwals. (the thinnest thing I have tried is WD-40 and I find that I don't care for it much) has anyone tried it on a Lynn idwal?
Sent from my SAMSUNG-SM-G935A using Tapatalk
-
01-27-2017, 05:39 AM #12Senior Member

- Join Date
- Nov 2012
- Location
- Seattle,WA.
- Posts
- 579
Thanked: 55
This doesn't make sense to me. Soap breaking the surface tension, it would seem to me, provides greater contact with the hone and therefore should result in a coarser finish.
Glycerin, it seems to me, provides more viscosity and thus results in less contact and therefore a finer finish.
What am I missing?
It's the surface tension aspect that doesn't make sense to me if you are talking about breaking the surface tension of the water.
I agree that thick lather is more viscous than is glycerin. It's the bringing of surface tension of the water into this that doesn't make sense to me. I don't think that's a factor here.Last edited by gcbryan; 01-27-2017 at 05:46 AM.
-
01-27-2017, 07:23 AM #13Previously lost, now "Pasturized"


- Join Date
- Oct 2005
- Location
- Winnipeg Manitoba Canada
- Posts
- 1,333
Thanked: 351
Oil and grease do not reduce surface tension on water.
Glycerine, soap and other surfactants do reduce surface tension on water
Glycerine can add viscosity to water, but it must first reduce surface tension in order to become emulsified with the water.
You ARE correct that surface tension is reduced by surfactants such as soap, but there is also hydroplaning in play which makes this whole question quite messy.
It is difficult to ascribe fixed results to these honing mediums, they rely on many other factors, such as speed of honing, pressure on the bevel while honing, the hone itself (porous, surface texture, scratches that might funnel water away from underneath the bevel while honing and so on) so in the end we really don't know for absolute sure what is going on and cannot say for certain that THIS+THAT=WHAT WE WANT no matter who does the honing and on any hone of that type.
We can only remind each other of things we have tried so they can try it and see if it does something desirable for them.
I find it quite easy to come up with a good edge, but those *perfect* edges are something else!"Aw nuts, now I can't remember what I forgot!" --- Kaptain "Champion of lost causes" Zero
-
01-27-2017, 08:55 AM #14Senior Member

- Join Date
- Nov 2012
- Location
- Seattle,WA.
- Posts
- 579
Thanked: 55
The whole concept of either using glycerin or just using plain water is to increase viscosity with glycerin so there is less contact with the hone.
If lather works, then that whole concept is to increase viscosity and reduce contact with the hone.
None of this has anything to do with using a minute amount of soap as a surfactant to reduce the surface tension of the water. That would make for a harsher result if this was a significant factor.
If reducing the surface tension of the water was a positive thing then we would just not use water in the first place.
-
01-27-2017, 04:01 PM #15illegitimum non carborundum



- Join Date
- Jan 2008
- Location
- Rochester, MN
- Posts
- 11,552
- Blog Entries
- 1
Thanked: 3795
Semantics is part of the problem here...
The term "surface tension" has been used around here for quite a while. It is not quite an accurate term for discussion of water on hones. A more accurate term is "wettability."
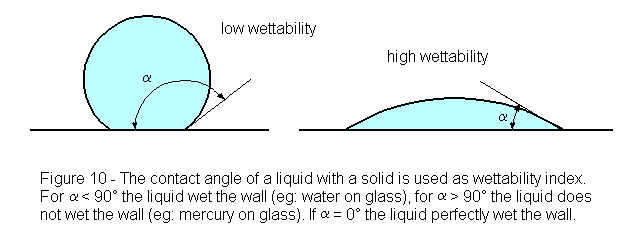
Some hones, because of their more glass-like surface, cause water to bead up on the hone rather than spreading as a uniform film over the honing surface. When honing with such beaded water, you are basically honing on a dry surface and the blade tends to push the beaded water right off of the hone.
The point of adding something to the water to increase wettability, or decrease surface tension, is to keep the water on the hone so that the serves as a lubricant between the hone and the blade. It has nothing to do with viscosity.
-
01-27-2017, 04:14 PM #16
-
01-28-2017, 06:42 AM #17< Banned User >

- Join Date
- Oct 2016
- Location
- Saratoga, CA
- Posts
- 597
Thanked: 59
Ok, layman's term you want. The slime factor. Engines use oil to reduce wear. Does it eliminate wear? No, of coarse not. But the effect is real and unavoidable. Some wear will occur, with which in an engine is not desireable, but when honing, it is... less is more.
Last edited by Aerdvaark; 01-28-2017 at 06:44 AM.


 18Likes
18Likes LinkBack URL
LinkBack URL About LinkBacks
About LinkBacks






 Reply With Quote
Reply With Quote

