Results 1 to 7 of 7
Thread: PH of Alum?
-
01-27-2013, 02:23 PM #1Member

- Join Date
- Nov 2011
- Location
- York, UK
- Posts
- 82
Thanked: 4 PH of Alum?
PH of Alum?
Hi ,
Does anybody know the ph value of Osma's alum bloc? or any alum bloc?
I was talking to somebody about alum the other day and they asked me. Good question i thought i need to find the answer
Cheers MW.
-
01-27-2013, 03:02 PM #2Senior Member


- Join Date
- Jan 2011
- Location
- Roseville,Kali
- Posts
- 10,432
Thanked: 2027
Is very acidic I would guess a PH of 5ish
-
01-27-2013, 03:05 PM #3Member

- Join Date
- Jan 2013
- Location
- sweden
- Posts
- 42
Thanked: 1
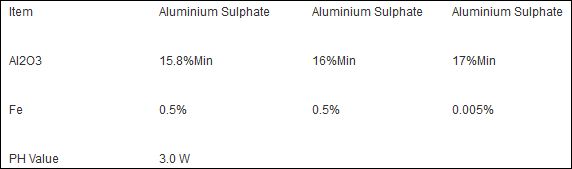
alum or aluminium potassium sulphate is acidic, lick on the bloc and you will taste it. When you put it on your skin it need to disolve with water to give the effect you want, I dont know exactly the % of the solution but if its 10% the pH is 3-3,5. I do not know the pH of the solid alum.
Last edited by Calle; 01-27-2013 at 03:05 PM. Reason: I wrote 3-2,5 instead of 3-3,5
-
01-27-2013, 03:50 PM #4

Anyone remember this?
For coffee it's 5; for tomatoes it's 4;
While household ammonia's 11 or more.
It's 7 for water, if in a pure state
But rainwater's 6, and seawater is 8.
It's basic at 10, quite acidic at 2,
And well above 7 when litmus is blue.
Some find it a puzzlement. Doubtless their fog
Has something to do with that negative log.
-
01-29-2013, 11:47 AM #5

I would say it's fairly acidic as well.
If it's ever gotten into your moth after applying, you'll know what I mean!!
-
01-29-2013, 03:45 PM #6Senior Member



- Join Date
- Apr 2008
- Location
- Essex, UK
- Posts
- 3,816
Thanked: 3164
-
The Following 4 Users Say Thank You to Neil Miller For This Useful Post:
Lemur (01-29-2013), mjsorkin (01-29-2013), MoreWhisky (01-30-2013), Pyrateknight (01-29-2013)
-
01-29-2013, 03:51 PM #7


 3Likes
3Likes
 LinkBack URL
LinkBack URL About LinkBacks
About LinkBacks






 Reply With Quote
Reply With Quote