Results 1 to 10 of 55
Hybrid View
-
01-28-2014, 07:18 AM #1
 Living up to my username - an ongoing thread about Silver Steel
Living up to my username - an ongoing thread about Silver Steel
Abstract: Energy-Dispersive X-ray Spectroscopy (EDS) was evaluated as a method of testing steel for noble metal content. Two examples of early-mid 19th century razors of English origin, suspected of containing noble metal alloying agents, were subjected to EDS to determine surface elemental content. Results showed that the generic branded razor advertised as "real silver steel" contained no silver, but there is the possibility that the second contained up to 1% platinum by weight. Further analysis is needed to confirm the results.
---------------------------------------------------------------------------------
I've always been curious about the claims of "Silver Steel". I know of the experiments of Faraday, and the few razors he had made (partnered with Stodart - Voidmonster can expand on this), but what of the claims of the multitudes of Sheffield razors stamped with "Silver Steel", or some variant?
Well, let's say someone I know has access to some equipment. Namely, an electron microscope with energy-dispersive x-ray spectroscopy (EDS) capability. Basically, you fire electrons at a sample, and those electrons knock out core electrons in atoms; during this process, x-rays are generated. Each element has very specific binding energies of those electrons, which are reflected in the energies of the x-rays. That's leaving out a lot, but should be enough to understand the idea. So theoretically, this technique should be able to tell whether a sample contains a given element; namely, silver.
For those curious, here is what is basically going on:
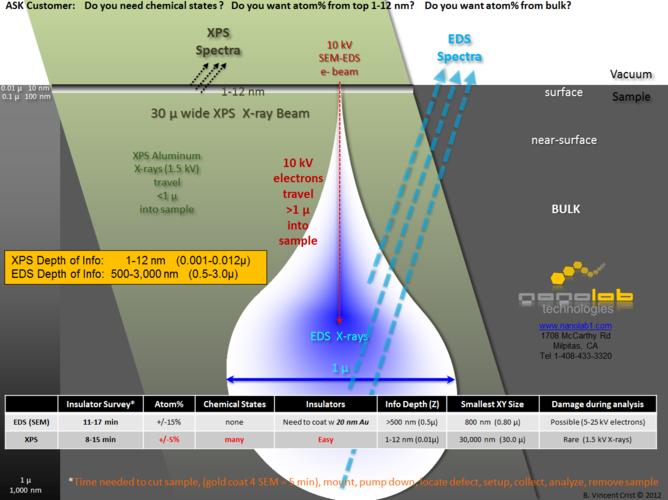
source: http://www.nanolabtechnologies.com
Now on to the fun stuff. The first up is a generic razor, no maker, marked only "Real Silver Steel":

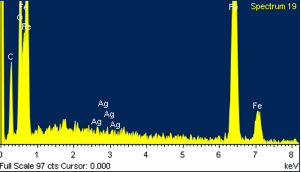
We'll see about that. Below are results from the EDS analysis. The first picture is what the spectrum looks like; where silver should be is marked 'Ag'. Samples were taken near the edge, as this is theoretically where any concentration of noble metal is likely to be, if present.
And here is the corresponding quantitative information:

This was repeated at multiple sites; Oxygen content is due to iron oxide, rust. Clearly, there is no silver in this steel. So we know we can't trust every razor that has such a stamping.
Edit: no silver in large concentration, but if the original recipe was 1/500, this technique with the specimen is not sensitive enough to determine the presence of silver.
Next up is a razor made from Pickslay's Peruvian Steel. This one:

For various reasons, which Voidmonster will be able to explain better than I, there is a good chance this steel actually contained platinum; in the recipe, it should have been around 1 percent by weight. Here are the results:
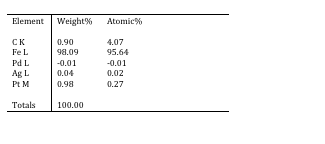
One thing to note is that you can tag any element that you want and it will 'look' for it, but just because the element is in the table doesn't mean it's in the sample. Anyway, what we see here is that, curiously, it looks like there's a good chance that this sample may contain ~1% platinum. This will need to be repeated to get the uncertainty in the measurement reported, as these numbers alone cannot be taken at face value with confidence, but they are interesting as a starter.
However, on this question, I think elemental analysis will be the way to go. It is a highly sensitive technique, although it is destructive, so it will require filings. The problem with the EDS results is that the noble metal alloying agents are in quite low (1-2 wt%) concentrations, so they will give a relatively low response. It is quite possible that the size of the background noise, relative to this response, could be interpreted as positive peaks when told to look for specific elements, so while the results seem tantalizing at first glance, they don't say much really when interpreted carefully. Further work is clearly needed.
But this is a good start I think. Hopefully others will experiment too! Add to this thread if you find anything interesting - history, documents, metallurgical recipes, elemental analysis results...Last edited by ScienceGuy; 01-28-2014 at 09:53 PM.
-
The Following 15 Users Say Thank You to ScienceGuy For This Useful Post:
Jimbo (01-28-2014), JimmyHAD (01-28-2014), KindestCutOfAll (01-31-2014), Martin103 (01-29-2014), MattCB (01-28-2014), Noswad (01-28-2014), Nspencer (01-28-2014), RoyalCake (01-28-2014), ScottGoodman (01-28-2014), spazola (01-28-2014), Steel (01-29-2014), Thisisclog (01-29-2014), Voidmonster (01-28-2014), WW243 (01-28-2014), Zephyr (01-28-2014)
-
01-28-2014, 02:42 PM #2

 ,,,,,,,what's an element ?
,,,,,,,what's an element ?
-
01-28-2014, 02:51 PM #3No that's not me in the picture

- Join Date
- May 2013
- Location
- Los Angeles South Bay
- Posts
- 1,340
Thanked: 284
Excellent work! This kind of stuff is very intriguing to me and have been tempted to have to the guys at work do some analysis - but have yet to bring in any donuts.
I love living in the past...
-
01-28-2014, 03:19 PM #4Senior Member


- Join Date
- Jan 2011
- Location
- Roseville,Kali
- Posts
- 10,432
Thanked: 2027
-
The Following User Says Thank You to pixelfixed For This Useful Post:
Hirlau (01-28-2014)
-
01-28-2014, 03:38 PM #5
-
01-28-2014, 04:44 PM #6

You all know seawater contains gold. It's just not commercially feasible to extract it. With concentrations so low I don't think those razors were meant to have PT or AG. They were just present in the ore with one of the alloys. If you tested for every known element you might be surprised with the result.
No matter how many men you kill you can't kill your successor-Emperor Nero
-
01-28-2014, 04:59 PM #7

Yes, but seawater concentration of Au is at best in the ballpark in the parts per million range. If the Faraday recipes were used it would be in the parts per hundred range, 4 orders of magnitude different. I agree that you'll find traces of many elements in any given sample, but if noble metals are present according to the supposed recipes, it should give a decent response. I found a couple rough analyses of iron ores and the later transition metal content was also in the ppm range, I don't think there is commercially used iron ore with parts-per-hundred concentrations of noble metals but would be interested in what you dig up.
-
01-28-2014, 05:18 PM #8Senior Member


- Join Date
- Jan 2011
- Location
- Roseville,Kali
- Posts
- 10,432
Thanked: 2027
Define Noble metals please
CAUTION
Dangerous within 1 Mile
-
01-28-2014, 08:06 PM #9
-
The Following User Says Thank You to edhewitt For This Useful Post:
Hirlau (01-28-2014)
-
01-29-2014, 07:51 PM #10


 86Likes
86Likes LinkBack URL
LinkBack URL About LinkBacks
About LinkBacks







 Reply With Quote
Reply With Quote







 see that was simple john.
see that was simple john.
