Results 21 to 30 of 45
-
06-20-2014, 04:33 PM #21Senior Member



- Join Date
- Apr 2008
- Location
- Essex, UK
- Posts
- 3,816
Thanked: 3164
-
06-20-2014, 05:36 PM #22
 !! Enjoy the exquisite taste sharpening sharpening taste exquisite smooth. Please taste the taste enough to ride cutlery.
!! Enjoy the exquisite taste sharpening sharpening taste exquisite smooth. Please taste the taste enough to ride cutlery.
Mike
-
The Following User Says Thank You to entropy1049 For This Useful Post:
Neil Miller (06-20-2014)
-
06-20-2014, 07:31 PM #23
-
06-21-2014, 12:28 AM #24

That has partially explained a few things to me.
What still mystifies me is what you are calling Activation Energy, and why some examples of celluloid reach the reversal stage before others, and why some are still showing no sign of reaching that stage.
Activation Energy! All molecules and the products composed of them have an amount of “free energy” within their structure. This free energy is the result of movement between the particles in the atoms in the molecules, most of which is from electrons bouncing around and bumping in to each other. The material is stable in this energy state because the free energy is countered by entropy, which is the natural tendency of matter to remain in its lowest energy state, and the two are happily engaged in a state of equilibrium. This is our celluloid happily in stasis, not decomposing. Now say we combine two of these stable molecules. If two of the molecules (our reactants) collide with sufficient kinetic energy, the molecules, not able to sustain themselves structurally beyond their free energy state, will engage in a reaction and a new molecule or molecules will be created from all or part of the reactants. Mixing sodium ion metal with chlorine gas will quickly result in table salt with no encouragement. With some reactants, insufficient kinetic energy (or geometry, or electrical charge, or time, or a number of other factors) prevents simply introducing the two together and obtaining a reaction. To these, we need to add supplemental energy to the reactants to overcome the free energy system. This is the Activation Energy. We can add it through a variety of mechanisms: mechanical agitation, UV excitement, heat, a new reactant, etc. This is what’s going to take our razor scales from their blissful, stable existence to their doom. The scales have no capacity to store additional energy over the years, so it would take a sudden introduction of sufficient activation energy to begin the decomposition reaction. So, if your celluloid is stable, and you are careful to never subject it to heat, light, water, or any of the other things we have in our shave dens (lol), you could hermetically seal it in a black, UV absorbing/non-passable container and put it in your refrigerator, and in six thousand years, should be able to open it and shave with your pristine celluloid scaled razor! Now, neither of us is going to take such totalitarian precautions, because we enjoy shaving with the damned things! So what we must do is try to limit these factors (exposure to UV light, high temps, soaking in water, etc.) that can stimulate this increase in activation energy. Some materials may act as catalysts in the decomposition of celluloid which would lower the activation energy required for reaction, speeding up the decomposition reaction in that manner, but I haven’t researched to see what these may be; Another day perhaps.
I used to belong to a group who made their own sensitised collodion by nitrating cotton in a nitric acid rich mix of nitric acid and sulphuric acid (horrible stuff as I am sure you know - one in particular is given to sending out clouds of vapour that spills down the sides of the flask), washing it copiously and then dissolving it in a mix of alcohol and ether to which some added a little chloroform. This was flowed onto a plate of glass and the whole dunked in an aquaeous solution of silver salts whle still tacky, the silver mx charmingly referred to as 'lunar caustic'. The mixes we used went back to the dawn of wetplate photography, which predated the marketable early varieties of celluloid/parkesine, and which shared many of the processes.
This is what any good wet chemist would call a Hot Time on a Saturday Night and would be considered a lot of fun by nerdy guys like me. You’re lucky you didn’t kill yourself <wink!>
In the recipe books of the time we have instructions for using the purest grade chemicals, which makes me wonder just how pure they were, particularly as the advice on obtaining clean water was to scoop it up from a puddle after it had rained for some time. Apart from that there were the effects of different level of nitration, which is determined by the strength of the acid and is of the formost importance in making gun-cotton. With those sort of amateurish attempts one can see that anything could have gone awry at any stage of the process, but for something like celluloid produced on a large-scale basis one would expect tighter quality control.
Purity of some reactants of the time would be dubious at best, I would imagine. Purifications can be tricky, even in modern times, and I can’t see some of these manufacturers having a lot of success in the synthesis of some of their reagents. This would definitely affect the resulting products of the reaction. Anything from a very, very dirty (contaminated by undesirable side products) to no reaction whatsoever would be the result. These contaminants could certainly cause problems and contribute to the more rapid decomposition of the material, especially if they were more reactive than the desired material. Here is where we find that, in addition to certain colored celluloids, some are simply more likely to decompose than others, based on these external manufacturing processes.
Gun Cotton! What fun! I once had a professor who used to say ”Whenever I look out into the lecture hall and see the kids are looking a bit glassy eyed, I know it’s time to blow something up”. Gun cotton was one of his weapons of choice. That, alkali metals, or super acids…
I know for instance that the stuff shrank a lot and I have seen a modern video of celluloid production, in Italy I think, where the sheets of celluloid were taken from the settling mould and stacked. This by sheer weight of pressure drove out a lot of fluid, and the stuff was turned and re-stacked for quite a long time (I forget how long now - definitely weeks, could have been months though) which got rid of the remaining free fluid and also allowed the material to shrink and become more dimensionally stable. How then did they incorporate metal mouldings and stampings like fancy razors into the scales? They look like they were placed in a mould and the celluloid mix injected on top, but then we would still have excess fluid in the celluloid and it would shrink a lot too, probably differentially at the junctions of the metal parts due to them having different rates of thermal expansion and contraction.
I don’t know for sure, but could only offer a supposition. At some point during the “setting” of the celluloid, it was determined its stability and malleability was in best proportion to either mold or press the pattern. Inlays were possibly hand-worked or possibly a weak solvent was used (much as we can set an inlay with acetone in the course of restoring one of our own razors) to make the material sticky and soft enough to be workable.
There is celluloid still in production in America - a lot of the knife-making companies sell it to make handle pieces and bolsters from, yet it no longer smells the same. There is not the merest hint of camphor (even back in the day though, camphor was not used for all types of celluloid, particularly the type that has an acetic acid or vinegary smell), instead it smells of the resin used to make glass reinforced plastic. I have queried this with one company, who assure me that the stuff is 100% celluloid - it looks like it, but I have my doubts.
One of the components of the structure of celluloid plastic is the nitration and polymerization of the camphor molecule. Any plastic not using camphor in its manufacture is some other type of plastic. This doesn’t mean something in the modern process hasn’t eliminated or retarded the decomposition process, however. Also, Caveat Emptor.
There is a niche market for it in Japan too, so it is not gone the way of the dinosaurs yet. In fact some luthiers insist on having the real thing to repair old guitars and the like, so there are other markets for it besides knife-making. One suspects that it still finds use in some high grade fountain pens and is milled into shapes like shaving brush handles, etc.
Funny, the effort to restore what was once considered a manufacturers “low-end” guitar (for example, a Martin D-18) can far exceed that expended to restore a higher end guitar that used rarer (at the time) natural materials like bone. I think Martin went to celluloid nuts and bridges for the lower end boxes immediately post WWII. I used to collect old Martins. I had to get out of that game…those babies get pricey. I’m sure there is a diverse niche market for the continued small scale production of celluloid plastic.
With regard to cell rot, the characteristic patterning caused by mottled celluloids is well known, but this sort of 'pattern staining' is seen on blades that have mottled scales not made of celluloid. Such a phenomenon as pattern staining is well known in the building industry - one example would be patching up the eaves of a building with different materials like thermal blocks, wood, metal and bricks and putting a render coat of plaster over the whole thing. Because of the difference in thermal load of the different products under the render coat, the air in contact with the wall is warmer in some places, colder in others. Air holds moisture, and when the temperature goes below a certain level it dumps that moisture - hence the misting up of windows and dew-line faults in buidings. The opposite is true to - where it is warmer the air moves more, so there is less chance of dumping its load (dirt/dust/water). After a while we begin to see the shadow of the the different material under the render.
I suppose that the same sort of thing can happen to razors - the lighter parts of the scales will let the metal warm up beneath them, while the darker bits will absorb that energy and leave the metal under then cooler. When the temperature drops we get a smaller-scale 'pattern-staining' effect.
This could well be the case. Also some light absorption could factor in? Both of these in a wet environment?
Does that affect celluloid scales that are breaking down? Is the leaching out of acid increased or delayed by this process? Is this why a controlled environment with no heat extremes is advised? Does keeping the items in the dark avoid the pattern-staining occurring in the first place by stopping the passage of light altogether?
Once the process is begun, I think it will proceed at its own pace unless the equilibrium is shifted in favor of decomposition by one of the factors we know to accelerate the process. The nitric acid will be synthesized stoichiometricly at the rate of the reaction. A dark, cool, unlit, dry environment is always going to be the safest environment for the preservation of our razors. The exclusion of light alone won’t completely protect our razors, as the other factors (heat, dampness, etc.) could still come into play as this dark material may be more susceptible to the decomposition process as a result of the added coloring and resulting adjuncts.
Darker, opaque celluloid scales seem immune to this - is this the result of fillers, or the smaller ratio of cellulose as a lot of the weight of the scale is made up from fillers and colouring agents? Or is there another reason entirely?
This could well be as the coloring agents would account for some mass. Also, the article mentions some compounds were found to stabilize celluloid, among them a titanium salt, and Ti salts are typically green-blue-gray. Also, I would suspect UV light absorption to be a factor in the immunity of some colored celluloids.
That is the sort of stuff I thought I would have seen, as my viewpoint is from the restoration of razors. A bit like driving a car, really -I am not that interested in how the engine was composed of elemental particles and worked and bored, I just want to know where to stick the petrol, oil and water! That is a personal point of view, conflicting with many other peoples points of view, just like my point of view about celluloid conflicts with that of many other people. Either way, I don't care - we take what we want from something like these examples and then move on - it doesn't really matter, does it?! I couldn't take much - my loss.
I understand your viewpoint completely, and I hope my windy overly chemistry-centric answers don’t cause you poor health! But I do feel that if a person knows what these precipitating and accelerating factors are, why they are, and how they can be minimized or prevented, we all win the day.
Your explanations and clarifications are very elegant though, and it is a pleasure to read them. You are a very clever person and I'm glad to have met you, even if it is only over the ether (not the type used in collodion, I hasten to add!).
And you Sir, are a fine craftsman and historian (and not a bad judge of character ). I place myself at your disposal should any incredibly boring and superfluous chemical synthesis question come boring itself into your mind.
). I place myself at your disposal should any incredibly boring and superfluous chemical synthesis question come boring itself into your mind.
PS I am older than you, therefor I am grumpier! Bah! Humbug!
There’s more gravy than of grave to the likes of you!
That’s the sum total of my knowledge of the works of Dickens.
Regards,
Neil
And My Respects Sir-
Mike!! Enjoy the exquisite taste sharpening sharpening taste exquisite smooth. Please taste the taste enough to ride cutlery.
Mike
-
The Following User Says Thank You to entropy1049 For This Useful Post:
Neil Miller (06-21-2014)
-
06-21-2014, 01:01 AM #25Senior Member



- Join Date
- Apr 2008
- Location
- Essex, UK
- Posts
- 3,816
Thanked: 3164
Excellent, Mike. You should have produced your own razor-centric article!
In all sincerity,
Neil
-
The Following User Says Thank You to Neil Miller For This Useful Post:
entropy1049 (06-21-2014)
-
06-21-2014, 01:15 AM #26
-
The Following User Says Thank You to JimmyHAD For This Useful Post:
entropy1049 (06-21-2014)
-
06-21-2014, 02:53 AM #27

Different scientific disciplines will sometimes use different definitions for the same thing. This ensures we never completely understand what the other is saying and helps us to maintain animosity towards each other
 One way of describing entropy is the energy in a closed system unavailable to do work. When I retired, it seemed appropriate
One way of describing entropy is the energy in a closed system unavailable to do work. When I retired, it seemed appropriate  !! Enjoy the exquisite taste sharpening sharpening taste exquisite smooth. Please taste the taste enough to ride cutlery.
!! Enjoy the exquisite taste sharpening sharpening taste exquisite smooth. Please taste the taste enough to ride cutlery.
Mike
-
06-21-2014, 06:01 AM #28Senior Member




- Join Date
- Nov 2012
- Location
- Across the street from Mickey Mouse in Calif.
- Posts
- 5,320
Thanked: 1185
I think I have read this article before a year or so ago. Then I went out a bought a lot of acrylic sheets :<0) My Ducks will live on past me and I believe Bresnick and Perlson are smiling down on me. After all, it's the metal that counts and if they had the choice of material I do I am sure they would have used it.
Thanks for posting it. It brought about more to read.
And Neil. I think the brass was put in a mold and the material poured in. Although not impossible, setting brass in an injection mold would have been some task. I may be way off base in this assumption knowing only about modern types of liquid injection molding.Good judgment comes from experience, and experience....well that comes from poor judgment.
-
06-21-2014, 11:21 AM #29Senior Member



- Join Date
- Apr 2008
- Location
- Essex, UK
- Posts
- 3,816
Thanked: 3164
I used to think it was a pour-in or injection mould process, but there seems no way that the logos or the bolsters would have remained in position. Some logos are actually soldered to a pin that is they bent at right angles and fills the core of the scale, which mitigates against it being pressed in after the scale is pressed, but some are just metal foils that the cellulose seems to have flowed around. You find the same thing in the newer Solingen inlaid scales, and these are a hard plastic, not cellulose, so the technology is still being used and one would only have to delve in today's processes for an answer rather than in the murky depths of the past.
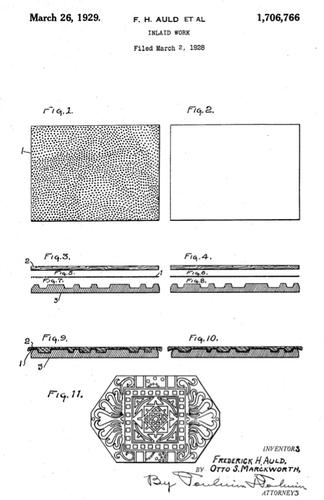
You never see any injection moulding ejector marks, either.
This makes Mike's suggestion that the stuff is pressed and then re-pressed again with the metalwork while it is still in an unset state. Maybe it was still tacky enough to hold the bolsters in place along with the metal foil before it was set back into the mould and pressed again.
The only thing I can find wrong with this is the shrinkage factor. I have seen the stuff made, and it shrinks enormously. The bolsters appear to be pre-drilled or pressed holes, made before attaching to the scales. If so they are gambling with the two scale halves shrinking at exactly the same rate and keeping more or less the same shape, otherwise the holes would not line up.
Sometimes the outer surface of the scale appears to have a finer, more polished appearance than the rest, which is only a few thou thick. This happens with other stuff that has been moulded too, a kind of laitance that migrates as a filmy, grain-free shiny matter to the junction of mould and material. You see it in cast concrete lintels and the like. This in itself shows that the metalwork has been in place during one pressing or another.
I used to think that perhaps they made a stick-like scale with the mouldings attached, then put this in the centre of the mould and flowed the cellulose around it, but you would expect to see evidence of this in a cross section, and all the broken celluloid scales along with ones I have broken intentionally did not support this theory either
Maybe the mould itself had 'stubs' that the pre-drilled holes in the metal bolsters fitted onto and which held them in place, but then we have to explain how the metal foil logo was kept in place. Maybe a thin solvent-rich coat of cellulose was dabbed onto the parts, like a thin glue, and this became absorbed into the celluloid once it was pressed.
Celluloid was a wonder material, used for all sorts of things, for instance buttons. Made to resemble ivory it gives us one of the earlier celluloid trade names of 'ivoroid' and like glass, metal inlays and fancy pieces were pushed into it while in a softened state. The surface would have to be refinished, of course.
Some attempts were made at partially dissolving the celluloid, but this trapped gases, solvents, etc and the liquified celluloid would not flow int the corners of the inlays because of this and trapped air. One company (see attachment - above: this shows a moulded fancy surface with an inly between two sheets of celluloid, but tthe top shhet could be just patterned or coloured celluloid) solved the problem by using two pieces of celluloid with the metal between them - the back layer of celluloid was perforated with minute holes, small enough to let solvents evaporate and trapped air to escape. However, the back would remain perforated, so it would have to be sealed in some manner. Indeed, some scales made of celluloid do seem to have a different layer at the back. I am looking at some cracked-ice Dubl Duk scales at the moment, and these have a back layer, thick enough to notice, which is homogeneous and appears quite different to the cracked-ice layer.
The answer is out there - somewhere!
Regards,
NeilLast edited by Neil Miller; 06-21-2014 at 01:14 PM.
-
06-21-2014, 01:02 PM #30

Perhaps sending an inquiry to Thomas @ Revisor ? Or maybe if anyone in the business has access, Heirbert Wacker ? Assuming someone working for Thomas, who was in the business way back when, or Herr Wacker , may recall the process used.
 Be careful how you treat people on your way up, you may meet them again on your way back down.
Be careful how you treat people on your way up, you may meet them again on your way back down.


 50Likes
50Likes LinkBack URL
LinkBack URL About LinkBacks
About LinkBacks








 Reply With Quote
Reply With Quote


