Results 11 to 20 of 30
Thread: Did i ruin my barbers hone?
-
08-03-2012, 02:39 PM #11

If its flat now , I would just use it as it is. It's the grit that you want, not a shinny surface. You lap them if they get shinny.
-
08-03-2012, 02:44 PM #12

Bad buzz! I have heard of this happening a few times for the reasons given (binder). If it was stored some place hot - that can do it!
I have been fortunate to never have received one, and look forward to hearing what becomes of yours as well. I'll just throw this out there though, a guy I know who had one that was falling apart, said he soaked his in vaseline that he heated up via bowl in hot water. It seemed counter intuitive to me, but he claimed it worked?
Good luck!David
-
08-03-2012, 04:57 PM #13Senior Member


- Join Date
- Apr 2008
- Location
- Saint Paul, Minnesota, United States
- Posts
- 2,944
Thanked: 433
I had the exact same thing happen to a two sided Norton (pike) hone, I never did find a solution
-
08-03-2012, 07:21 PM #14Senior Member

- Join Date
- Jul 2011
- Location
- Ponca City, Oklahoma
- Posts
- 605
Thanked: 66
same problem here with a swatty, I soaked it in water a few days, when I pulled it out, it's like talc powder now. completely not usable.
-
08-03-2012, 10:15 PM #15Member

- Join Date
- Jul 2012
- Posts
- 39
Thanked: 10
Ok. The hone is looking decent. First I lapped it at 320. It shed grit like crazy. Then I cut some canned shellac about 50% with denatured alcohol. I rinsed and dried the hone. Then I applied the finish as heavily as a semi saturated rag could do it. When that dried i hand scuffed the hone at 1200. Wiped it quickly with a rag with just alcohol. Let that tack up, and applied shellac just like the first coat. I did this whole thing 3 or 4 times. Now it has got a scratch pattern close to my coticule. The surface of the stone is not shiny, it just drank the finish up. I think that I will let it dry good overnight and try to re lap tomorrow. At this point i will give this process a 6 out of 10 for salvaging a hone. But who knows the thing could turn to crap in the morning. I will have to keep an eye on it before i suggest this to anyone. I did use it to touch up a razor which shaved nicely a few hours ago.
-
The Following User Says Thank You to JamesT For This Useful Post:
earcutter (08-03-2012)
-
08-03-2012, 11:28 PM #16

One of my made by them say to
get free of heat sources.
and keep out of direct sunlight.
-
The Following User Says Thank You to Suile For This Useful Post:
JamesT (08-04-2012)
-
08-04-2012, 12:03 AM #17Member

- Join Date
- Jul 2012
- Posts
- 39
Thanked: 10
Went out for one last shellacking. I lapped the hone at 600, almost burning clear through what I had stabilized earlier today. The shellac hadnt penetrated very deeply. Cut some new shellac a little more than 50% and applied. This stuff laid down really nicely and after it dried i was able to scuff with 1200, wipe with water, and burnish the surface with the stones own swarf, collected from the 600 paper. Wiped with water and the finish was pretty consistent and kinda looked foggy / waxy like a NOS hone!
Best news is its surpassed both my coticules, at least just looking at scratch pattern. Its still fast, too. I am going to quit while I am ahead.
So I found that I could revive a roached hone. However the new surface probably won't survive a lapping. Perhaps a prolonged soak in shellac, maybe cut to 25% would penetrate more. Im just happy to be able to use it! For now, experiment over.
-
-
08-04-2012, 12:01 PM #18Senior Member



- Join Date
- Apr 2008
- Location
- Essex, UK
- Posts
- 3,816
Thanked: 3164
Most of the barber hones you find were made using one of two methods. 'Hot' hones were baked to set them and 'cold' hones were left to set in the mould using a chemical reaction and no baking. An even earlier method made use of gelatin mixed with potassium dichromate as the binder. Gelatin gets 'gummy' but does not set hard - the purpose of the pot. di. was to make it light sensitive and harden it. The mixing was carried out in subdued or red/yellow safe lighting, then once poured in the moulds they were exposed to sunlight - the UV radiation in the sunlight caused the pot. di. to react with the gelatin and make it go very hard. I suspect that the presence of abrasive grit, fillers and colouring affected how deeply the gum was hardened though, which might explain why they get softer when lapped - I can't see this happening with the 'hot' variety of barber hones. I used to use pot. di. to make felt hats (it stiffens them) and gum-bichromate prints. However, it is a bit explosive and also a carcinogen.
One of the main problems encountered was the mixing of the exceedingly fine dry abrasive element with the binder. Too much binder and the hone shrank or cracked, too much powder and it wouldn't mix properly. I think this is due to some sort of 'thixotropic' phenomenon: the dry powder mixes well with the wet binder, but as the percentage of powder gets higher although the combination may flow if poured slowly it becomes hard when it encounters the force needed to mix it. You can do the same with cornflour - make it up to a thick consistency, maybe a bit looser than toothpaste, and you can pour it and it will spread out on the table. However, if you pour it in your hand and quickly form it into a ball it will hold its shape as if hard - then flow away again when you stop rolling it about. You can slowly push your finger into it in a glass til you touch the bottom, but if you use any force you can't penetrate it. Anyway, little balls of binder adhere around the mixer blade and gradually a blocky mass forms that is not well mixed. You can see the evidence of this in some barber hones - patches of lighter colour and places where poorly mixed bits have plucked out leaving little pits.
I dimly remember Russ's barber hone - it came from me, unfortunately, but we squared things up! - it was a black one I think and called something like 'Crow' or 'Raven' - might be wrong, though!
James - your shellac method reminds me of when I worked in May & Bakers lab as a young man - we used a process somewhat similar. The article to be infused with preservative (usually some sort of insect to mount on a slide) was totally immersed in alcohol alone for a week. This got the water out of it. Then it was immersed in a fresh alcohol bath for some days, followed by being immersed in a 5% solution of the mountant in alcohol. This continued in stages - say 25%, 50%, 75% and then full strength. The idea was to draw the medium right down into the specimen. Sometimes we used resins like canada balsam and something like xylene or toluene as the thinner, but these are now both regarded as carcinogens.
Regards,
NeilLast edited by Neil Miller; 08-04-2012 at 12:10 PM.
-
-
08-04-2012, 02:26 PM #19Hones & Honing



- Join Date
- May 2005
- Location
- Saint Paul, Minnesota, United States
- Posts
- 8,023
- Blog Entries
- 1
Thanked: 2209
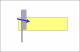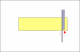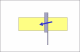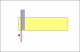
I see that the hone is stamped "Olean, New York" . It was most likely made by the American Hone Co. If so then they used 2 very different types of binders and very different processes to make each type of hone.
What I suspect is that the hone was subjected to either or both of extreme cold ( freeze/thaw cycle) or high heat temps. The freeze/thaw cycle would most likely break down both types of binder and the high heat exposure would breakdown a resin binder.
Based on your pic the join line of the 2 halves appears to be a "earth type" binder, not a resin, but the assembly process used in Olean may not be the same as was used in Moravia. Since I know the ingredients and processes used by that company I can say with certainty that there is no way to "reconstruct" that hone to a like new condition. Except, you might try putting the hone in an oven at a temp of 125F-150F for about 2-3 hours. If it is a earth based hone then that just might help. But if it is a resin based hone it may harm it even more. Sorry.
Does anyone know of a chemical test for clay?Last edited by randydance062449; 08-04-2012 at 02:33 PM.
Randolph Tuttle, a SRP Mentor for residents of Minnesota & western Wisconsin
-
-
08-04-2012, 03:54 PM #20Senior Member



- Join Date
- Apr 2008
- Location
- Essex, UK
- Posts
- 3,816
Thanked: 3164
That's a hard one, Randy - there is more than one type of clay! Clays are a subclass of silicates, known as phyllosilicates and there are four broad divisions. To do a test you would have to extract the clay content, which is hard to do. A simple field soil test might be an answer. In it the sample is soil which contains sand, loam, silt and clay. You get a sample, pulverise it, fill a tall narrow bottle up to the 1/4 mark with the sample and then fill to the 3/4 mark with distilled water. Add a small amount of non-foaming detergent, shake for 10 mins and place the bottle where you will not need to move it for about 3 days (or more). The first layer to drop out will be the sand - should begin to fall out right away and be complete in around 10 - 12 mins. Next will be silt and loam - completed in around 24 hours. Last will be the clay - the water will not clear for at least 2 or 3 days, sometimes in around a week. If you knew what the other additives were and how much mass they had you could plot if anything would tend to fall out after clay.
The trouble with clay is the bond made by the crystals. They are like a series of cages, consisting of sheets bonded to other sheets. In some the bonding area is filled with water. This type has the ability to lose water and to take it on again, cycling between swelling and heaving. It affects anything else incorporated in it - it can loosen other particles from the binder and if sufficient water is taken on it becomes plastic, deforms, and weakens the whole structure made with it. The other type has stronger bonds and is called 'inert' as it does not shrink and heave regardless of whether there is water present or not.
If you know for a fact that clay has been used, and the locality it came from, then you can ascertain from a geological survey map whether the clay is harmful or inert. For instance, clay found in weathered basaltic rock formations swells and shrinks, whereas greywacke rocks produce good, inert clay.
If you can isolate the clay - perhaps using the simple soil test above, then you can use methylene blue dye to see what type it is. You put some of the sample in a dish, add distilled water, swish it about and add methylen blue drop by drop and leave it to stand for a couple of hours (I think - it could be longer). If you have a microscope, after having poured off the liquid and washed out the extraneous dye, look at it under the lens and if the dye is seen in large amounts it is the harmful type. If only a tiny bit is stained it is the inert type.
You could also sieve your sample to find out. Clay is usually considered to be made up of particles of less than 0.002mm in size, so anything passing a sieve of this mesh would be clay. I suspect you would have to wet-sieve and constantly agitate the sieve, though. If you know nothing else in the mix is around this size, then I suppose that would be an easy way - but not as easy as the soil test.
Regards,
Neil
-


 20Likes
20Likes LinkBack URL
LinkBack URL About LinkBacks
About LinkBacks






 Reply With Quote
Reply With Quote
